For the last ten years the authors have been looking for correlates of their two-component model of long-term memory with up-to-date findings in the field of molecular memory (Wozniak and Gorzelanczyk, 1994; Wozniak et al., 1995, Gorzelanczyk and Wozniak, 1996, Gorzelanczyk and Wozniak, 1997). The two-component model of long-term memory originates from the author’s findings in reference to repetition spacing in learning (Wozniak et al., 1995). The optimum repetition spacing indicates the existence of two independent variables needed to describe the state of long-term memory at any given time. These variables have been named retrievability and stability and have the following interpretation:
- retrievability determines the probability of recall of a given memory engram at a given moment and can loosely be associated with what is commonly called the synaptic strength (Abraham and Tate, 1997; Frey and Morris, 1997)
- stability is a less-studied property of memory which determines the speed with which retrievability declines after training. In other words, stability determines the rate of forgetting (Wozniak et al., 1995)
Although the speed at which the research field of molecular aspects of memory is truly hard to keep up with, some of most fundamental assumption related to our molecular two-component model of memory have not been changed.
It has been increasingly well documented in recent years that synaptic plasticity, a physiological basis of learning and memory, can mainly be classified into two categories: 1) relatively short-term changes in electrical activities and 2) more long-lasting morphological changes in synapses (Jodar and Kaneto, 1995). The authors conclude that these findings correlate well this their two component model of memory in which retrievability is correlated with synaptic conductivity while stability is correlated with lasting changes in synaptic membrane structure (Gorzelanczyk and Wozniak, 1996; Gorzelanczyk and Wozniak, 1997).
Let us first take a short look at the most interesting processes occurring in a conditioned synapse:
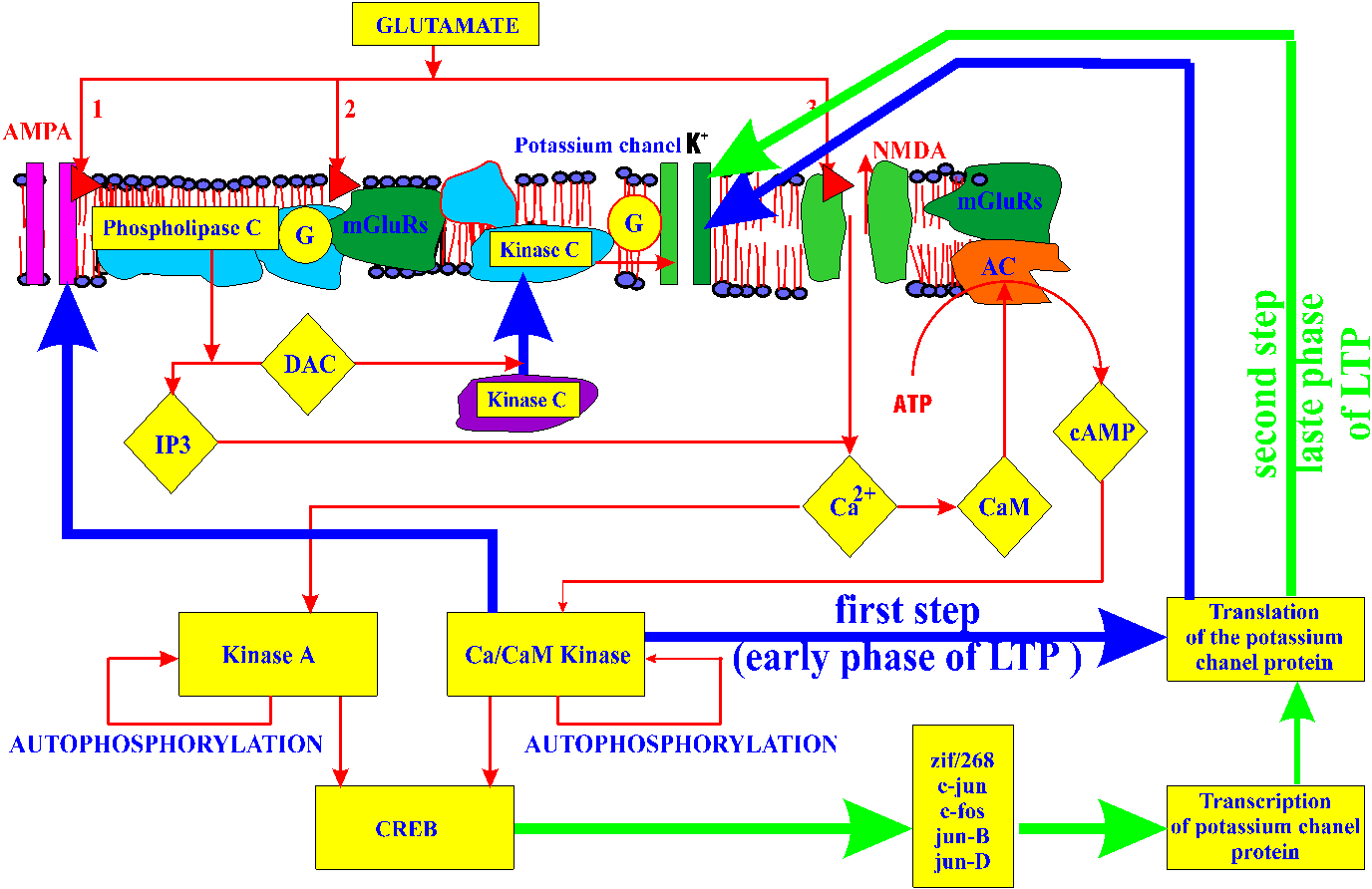
- as a result of releasing the neurotransmitter glutamate into the synaptic cleft, glutamate receptors are activated. In particular, (1) NMDA receptor whose activation results in an inflow of calcium into the postsynaptic element (Miyamoto and Fukunaga, 1996), and (2) metabotropic glutamate receptor (mGluR)(glutamate is here a leading neurotransmitter of interest due to its role in eliciting LTP in the mammal hippocampus) (Anwyl, 1994; Riedel and Reymann, 1996; Dube and Marshall, 1997)
- a series of intracellular
kinases is activated:
- mGluR coupled with protein G (Chou and Lee, 1995; Parmentier et al., 1996) activates phospholipase C that in turn releases diacylglycerol (DAG) from phosphatidylinositol 4,5-biphosphate (Thomas, 1995 in et al., 1997). DAG activates protein kinase C (PKC) (Riedel et al., 1996) and triggers its migration from the cytosol to the membrane (probably via proteolytic change to the enzyme affecting its hydrophobic properties (Apel et al., 1990; MacNicol, 1992). This transition is critical as it will ultimately result in phosphorylation of potassium channels (Muller et all., 1992) and increased potentiation of the synapse (Meiri et al., 1997)
- calcium flowing into the cell as a result of depolarization and a result of its release from intracellular stores takes part in activation of calmodulin-dependent CAM kinase II (CAMKII) (Kelly, 1991 Muller et all., 1992; Wayman et al., 1996) which can persist in active state due to autophosphorylation (Strack et al., 1997)
- calcium exerts its effects via calmodulin also on adenyl cyclase (AC) (Choi et al., 1992a; Choi et al., 1992b) that leads to increased levels of cAMP that migrates to the cell nucleus, activates protein kinase A (PKA) and triggers gene expression (Hagiwara et al., 1993; Motminy, 1997)
- at the same time, in a less well understood process, synaptic activation triggers the creation of a short-lasting, protein-synthesis-independent synaptic tag which later will take part in sequestering relevant proteins needed to establish further stages of memory consolidation (Frey and Morris, 1997)
- activating kinases CAMKII and PKA results in the expression of cyclic AMP-response element binding protein (CREB) (Montiminy, 1997; Kogan et al., 1997)
- CREB triggers a gene expression cascade with a group of IEGs coding for transcription factors such as zif/268, c-fos, c-jun, junB, and junD that in turn lead to transcription of late effector genes coding for proteins critical for establishing long-term memory (Abraham et al., 1991; Moore et al., 1996)
- a number of new proteins are synthesized including: ubiquitin hydroxylase (which may cause permanent proteolitic activation of PKA) (Hedge et al., 1997), clathrin (involved in membrane protein complexes (Solomonia et al., 1997), calreticulin (calcium-binding protein of the lumen of the endoplasmic reticulum) ( Kennedy et al., 1992), ependymin (protein involved in the axon growth and exhibiting ion-dependent polymerization) ( Schmidt et al., 1995), TPA (protease that may also be involved in membrane rebuilding) (Qian et al., 1993), protein G (Riedel et al., 1996), mGluR, microfilament proteins, gephyrin (receptor/channel clustering molecule) (Kawasaki et al., 1997), cell adhesion molecules (NCAM) (Sheppard et al., 1991; Sharp et al., 1993; Doyle and Regan, 1993), fasciclin II, BiP (reticulu m-resident protein involved in the folding and assembly of secretory and membrane proteins) (Sharp et al., 1993), and more
From the very beginning of our quest, it was clear that retrievability must be established in relatively short period of time following the training (Wozniak and Gorzelanczyk, 1994). We focused our interest on phosphorylation of the potassium channels as the best studied and most tangible factor affecting the synaptic potentiation after training (Premkumar and Ahern, 1995; Etcheberrigaray et al., 1996; Meiri et al., 1997).
Similarly, we thought of stability as of a factor that had to durably affect the speed of potassium channel dephosphorylation and be specifically related to the synaptic site (Premkumar and Ahern, 1995). This specificity and durability lead us to focusing on membrane protein complexes. Most of all, the list of proteins that have been found involved in memory consolidation seems to strongly reaffirm this conviction. Note, for example, that BiP might serve to fold proteins and assemble protein complexes necessary for the structural changes characteristic of long-term memory (Sharp et al., 1993). Those changes, according to our model, should reversely affect the rate of potassium channel dephosphorylation (Toral et al., 1994; Marom and Abbott, 1994).
In other words, we believe that the following components of the above molecular picture correlate with the two components of long-term memory:
- Retrievability - phosphorylation of potassium channels
- Stability - stability of membrane protein complexes involved in neurotransmission
In our earlier publications, we have listed nine properties of the two component-model of long-term memories and suggested that these be used in looking for molecular correlates of retrievability and stability (Wozniak et al., 1995). Here we shortly list those properties again with a view to establishing their congruence with the current research findings and the assumed correlates of retrievability and stability.
- R should be related to the
probability of recalling a given memory engram; forgetting
should be understood as the decrease in R
- Indeed, the greater the phosphorylation, the lower the flow of potassium (Alkon et al., 1991; Premkumar and Ahern, 1995), the greater the excitability of the membranes, the greater the probability of recall.
- R should reach a high value
as early as after the first repetition, and decline rapidly
in the matter of days (the average optimum inter-repetition
interval for retention 95% equals several days)
- Indeed, phosphorylation builds up very quickly and seems to be equally volatile.
- S determines the rate of
decline of R (the higher the stability of memories, the
slower the decrease of retrievability)
- This property cannot be verified. Stability of the membrane protein complexes should somehow affect the stability of potassium channel phosphorylation; perhaps by slowing down the reverse migration of PKC to the cytosol.
- With each repetition, as S
gets higher, R declines at a slower rate (stability of memory
increases with successive repetitions)
- This has not been unambiguously verified, but seems to be quite natural. With additional training, more protein synthesis occurs and the complexes grow will probably grow in strength.
- S should assume a high
value only after a larger number of repetitions (stability of
memories is positively correlated with the amount of
training)
- This point is a direct consequence of Point 4 that can effectively be used to search for proteins involved in membrane stability. We could differentially look for proteins that increase in concentration or activity upon repeated exposure to training.
- S should not change
(significantly) during the inter-repetition interval
- This is a weak proposition; not entirely critical for the validity of the two component model of memory. Neither has it been verified. However, protein complexes are the most likely candidate for durable changes in the synapse.
- R and S increase only as a result of an repetition
- Indeed, both the phosphorylation of potassium channels and the synthesis of new proteins come in the wake of training.
- If the value of R is high,
repetitions do not affect S significantly (this property is
implied by the spacing effect observed in learning)
- Not verified. As PKC migrates to the membrane, its cytosolic availability declines. This might result in depressing its ability to take part in inducing gene expression.
- As S increases, its further
increase becomes easier and easier
- Not verified. The membrane complexes themselves might somehow be involved in eliciting gene expression, e.g. by facilitating the increase in cAMP levels. Perhaps, stability is correlated with the number of mGluR receptors in the membrane while mGluR is involved in regulating cAMP levels and consequently gene expression.
If you have comments that could enhance our knowledge of the aforementioned phenomena,please write to Piotr Wozniak or Edward Gorzelanczyk.
See also:
References
- Abraham W. C., Dragunow M., Tate W.P, The role of immediate early genes in the stabilization of long-term potentiation. Mol. Neurobiol. 1991; 5(2-4): 297-314
- Abraham W.C., Tate W.P. Metaplasticity: a new vista across the field of synaptic plasticity. Prog. Neurobiol.,1997; 52(4), 303-23
- Alkon D. L., Matzel L. D., Collin C. Molecular mechanisms of memory and drug dependence. Alcohol-Alcohol-Suppl. 1991; 1: 35-7
- Anwyl R. Synaptic plasticity. A molecular switch for memory. Curr. Biol., 1994, 1; 4(9): 854-6
- Apel, E. D., Byford, M. F., Au, D., Walsh, K. A. and Storm, D.R. ,1990, Identification of the protein kinase C phosphorylation site in neuromodulin. Biochemistry 29: 2330-2335.
- Bullock S., Steven P. R., Pearce B., Potter J. Training Chics on a Passive Avoidance Task Modulates Glutamate-stimulated Inositol Phospate Accumulation. Eur. J. Neurosc., 1992, 5, 43-48.
- Choi, E. J., Wong, S. T., Hinds, T. J. and Storm, D. R. (1992a) Calcium and muscarinic agonist stimulation of type I adenylyl cyclase in whole cells. J. Biol. Chem. 267: 12440-12442.
- Choi, E. J., Xia Z., and Storm D.R. Stimulation of the type III olfactory adenylyl cyclase by calcium and calmodulin. Biochemistry 1992b,31:6492-6498.
- Chou J. C., Lee E. H. Differential involvement of hippocampal G-protein subtypes in the memory process of rats. Neuroscience. 1995; 64(1): 5-15
- Doyle E., Regan C. M. Cholinergic and dopaminergic agents which inhibit a passive avoidance response attenuate the paradigm-specific increases in NCAM sialylation state. J. Neural. Transm-Gen. Sect. 1993; 92(1): 33-49
- Dube G. R. Marshall K. C.Modulation of excitatory synaptic transmission in locus coeruleus by multiple presynaptic metabotropic glutamate receptors. Neuroscience. 1997; 80(2): 511-21
- Etcheberrigaray R., Payne J. L., Alkon D. L. Soluble beta-amyloid induces Alzheimer's disease features in human fibroblasts and in neuronal tissues. Life-Sci. 1996; 59(5-6): 491-8
- Frey U. Morris R. G. Synaptic tagging and long-term potentiation Nature. 1997, 385(6616): 533-6
- Gorzelanczyk E. J., Wozniak P. A. A new conceptual model of the processes involved in the formation of memory traces in the synapse. J. Neurol., 1997, 244, 139.
- Gorzelanczyk E. J., Wozniak P. A. New approach to studying molecular memory based on understanding the optimum repetition spacing in learning J. Neurol. 1996, 3, 5.
- Hagiwara, M., Brindle, P., Harootunian, A., Armstrong, R., Rivier, J., Vale, J., Tsien, R. and Montminy, M. R. Coupling of hormonal stimulation and transcription via the cAMP responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol. and Cell Biology, 1993 13, 4852-4859.
- Hedge A. N., Inokuchi K., Pei W., Casaido A., Ghirardi M., Chain D. G., Martin K. C., Kandel E. R., Schwartz J. H. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia.Cell. 1997; 89(1): 115-26
- Jodar L., Kaneto H. Synaptic plasticity: Strairway to memory. Jpn. J. Pharmacol.1995, 68, 359-387
- Kawasaki B. T., Hoffman K. B., Yamamoto R. S., Bahr B. A. Variants of the receptor/channel clustering molecule gephyrin in brain: distinct distribution patterns, developmental profiles, and proteolytic cleavage by calpain. J-Neurosci-Res. 1997; 49(3): 381-8
- Kelly P. T. Calmodulin-dependent protein kinase II. Multifunctional roles in neuronal differentiation and synaptic plasticity. Mol. Neurobiol. 1991; 5(2-4): 153-77
- Kennedy T. E., Kuhl D., Barzilai A., Sweatt J. D., Kandel E. R. Long-term sensitization training in Aplysia leads to an increase in calreticulin, a major presynaptic calcium-binding protein. Neuron. 1992; 9(6): 1013-24
- Kogan J. H., Frankland P. W., Blendy J. A., Coblentz J., Marowitz Z., Silva A. J. Spaced training induces normal long-term memory in CREB mutant mice. Curr-Biol. 1997, 7(1): 1-11
- Lin F. F., Varney M., Sacaan A. L., Jachec C., Daggett L. P., Rao S., Flor P., Kuhn R., Kerner J. A., Standaert D., Young A. B., Velicelebi G. Cloning and stable expression of the mGluR1b subtype of human metabotropic receptors and pharmacological comparison with the mGluR5a subtype. Neuropharmacology. 1997 Jul; 36(7): 917-31
- MacNicol, M., and Schulman, H. Cross-talk between protein kinase C and multifunctional Ca2+/calmodulin-dependent protein kinase. J. Biol. Chem. 1992, 267:12197-201
- Marom S., Abbott L. F. Modeling state-dependent inactivation of membrane currents. Biophys-J. 1994 Aug; 67(2): 515-20
- Meiri N., Ghelarandi C., Tesco G., Galeotti N., Dahl D., Tomsic D, Cavallaro S., Quattrone A., Capaccioli S., Bartolini A., Alkon D. L. Reversible antisense inhibition of Shaker-like Kv1.1 potassium channel expression impairs associative memory in mouse and rat. Proc-Natl-Acad-Sci-U-S-A. 1997 Apr 29; 94(9): 4430-4
- Miyamoto E., Fukunaga K. A role of Ca2+/calmodulin-dependent protein kinase II in the induction of long-term potentiation in hippocampal CA1 area. Neurosci.Res. 1996 Jan; 24(2): 117-22
- Montiminy M.Transcriptional regulation by cyclic AMP. Annu-Rev-Biochem. 1997; 66: 807-22
- Moore A. N., Waxham M.N., Dash P. K. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J-Biol-Chem. 1996 Jun 14; 271(24): 14214-20
- Motminy M. Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 1997; 66: 807-22
- Muller W., Petrozzino J. J., Griffith L. C., Danho W., Connor J. A., Specific involment of Ca2+ calmodulin kinase II in cholinergic modulation of neural responsivness. J. Neurophysiol., 1992, 68, 2264-2269.
- Parmetier M. L., Pin J. P., Bockaert J., Grau Y. Cloning and functional expression of a Drosophila metabotropic glutamate receptor expressed in the embryonic CNS. J. Neurosci., 1996, 16(21): 6687-94
- Premkumar L. S., Ahern G. P. Blockade of a resting potassium channel and modulation of synaptic transmission by ecstasy in the hippocampus. J-Pharmacol-Exp-Ther. 1995 Aug; 274(2): 718-22
- Qian Z., Gilbert M. E., Colicos M.A., Kandel E.R., Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature 1993, 361(6411):453-457
- Riedel G., Reyman K. G. Metabotropic glutamate receptors in hippocampal long-term potentiation and learning and memory. Acta. Physiol. Scand. 1996, 157(1), 1-19
- Riedel G., Wetzel W., Reymann K. G. Comparing the role of metabotropic glutamate receptors in long-term potentiation and in learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1996; 20(5), 761-89
- Riedel G., Wetzel W., Reymann K. G. Comparing the role of metabotropic glutamate receptors in long-term potentiation and in learning and memory. Prog-Neuropsychopharmacol. Biol. Psychiatry. 1996, 20(5): 761-89
- Schmidt R., Brysch W., Rother S., Schlingensiepen K. H. Inhibition of memory consolidation after active avoidance conditioning by antisense intervention with ependymin gene expression. J. Neurochem. 1995, 65(4): 1465-71
- Sharp-FR; Hisanaga-K; Sagar-SM NMDA receptor blockade prevents translation, but not transcription, of the c-fos gene following stimulation with multiple extracellular signals in cultured cortical neurons: implications for plasticity and molecular memory. NIDA-Res-Monogr. 1993; 125: 172-80
- Sheppard A., Wu J., bahr B. A., Lynch G. Compartmentation and glycoprotein substrates of calpain in the developing rat brain. Synapse. 1991, 9(3): 231-4
- Solomonia R. O., McCabe B.J., Jackson A.P., Horn G. Clathrin proteins and recognition memory. Neuroscience, 1997, 80(1):59-67
- Strack S., Choi S., Lovinger D. M., Colbran R. J. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J. Biol. Chem., 1997, 23; 272(21): 13467-70
- Thomas R. J.Excitatory amino acids in health and disease. J.Am.Geriatr.Soc. 1995 Nov; 43(11): 1279-89
- Toral J., Hu W., Barrett J. E., Sokol P.T., Ziai M. R. Use of cultured human neuroblastoma cells in rapid discovery of the voltage-gated potassium-channel blockers. J. Pharm. Pharmacol. 1994; 46(9): 731-4
- Wayman G. A., Wei J., Wong S., Storm D. R. Regulation of type I adenylyl cyclase by calmodulin kinase IV in vivo. Mol-Cell-Biol. 1996 Nov; 16(11): 6075-82
- Wozniak P. A., Gorzelanczyk E. J. Optimization of repetition spacing in the practice of learning Acta Neurobiol. Exp. 1994, 54, 59-62.
- Wozniak P. A., Gorzelanczyk E. J., Murakowski J. A. Two components of long-term memory. Acta Neurobiol. Exp. 1995, 55, 301-305